Migraine – the hidden public health issue
Migraines are often characterised by severe headache, but can manifest several other symptoms such as sensory disturbances and nausea. They are sometimes preceded by warning symptoms in the form of visual disturbances, feelings of numbness, speech difficulties or mild paralysis. Migraine attacks typically last from four hours to three days. The headaches are severe, pounding and often located on one side of the head. The side that is affected can change from time to time, and even during an ongoing attack. Migraine attacks are often accompanied by nausea, vomiting and sensitivity to light and sound.
According to the WHO, around 12 percent of the world's population suffer from recurring migraines. The real figure is larger, however, as the problem is both underdiagnosed and undertreated. The illness is more common in women than in men, and occurs in varying degrees in different age groups. The highest proportion is seen in women around the age of 40, with almost a quarter experiencing recurring problems with migraines1.
Migraine is a neurological illness. The precise cause has not yet been determined, although it is known that the headache during a migraine attack is caused by an expansion of the blood vessels surrounding the brain. Factors that can trigger an attack include stress, hormonal changes, hypersensitivity to certain foods, bright lights and strong smells.
A billion-dollar market – in flux
The global market for prescription medication for treating migraine amounted to around USD 3.7 billion2 in 2013. The world market is currently dominated by medications based on triptans, which make up around 85 percent of all prescribed migraine3 medication.
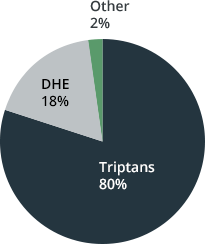
In terms of geographical markets, the USA has a special status. The US market is responsible for around 80 percent of the global market. Triptans make up around 80 percent here. In the USA, medications based on DHE (dihydroergotamine) are also used. This segment is responsible for around 18 percent of the market3.
Both triptans and DHE have a contracting effect on the blood vessels that have expanded in an uncontrolled manner. As a result, the blood vessels are restored to a more normal state, while the substances also impede the release of anti-inflammatory peptides.
Triptans and DHE work in different ways on different members of the serotonin 5-HT receptor family. In the USA, DHE is often given to patients who do not respond to triptans or those who have an existing cardiovascular illness. This particular patient segment is expected to increase significantly in the years to come, in line with the increased occurrence of cardiovascular diseases3.
For both of these categories, the patents behind the medications that have dominated the market to date have expired, which is opening the way to new players who, through generic drugs and innovation, can offer new concepts with improved patient benefits.
| Substance | Triptan* | DHE |
|---|---|---|
| Proportion of sales value | 80 percent | 18 percent |
| E.g. the brands | Imigran®, Zomig®, Maxalt® | Migranal® |
| Form of distribution | Tablet, injection, nasal spray | Injection, nasal spray |
| Patent situation | Generic drugs possible on a global basis | Generic drugs possible on a global basis |
* Sumatriptan, Naratriptan, Zolmitriptan, Rizatriptan, Almotriptan, Eletriptan, Frovatriptan
Triptans
Around 80 percent of the total global market for treating migraines is made up of medications based on the active substance triptan. Triptans are a collective name for a group of medications that contract the blood vessels in the event of a migraine attack. Triptans act on the blood vessels in the brain via 5HT1B and 5HT1D receptors, which contract the vessels and thereby stabilise the blood flow. They act against both the headache and other symptoms, such as nausea and sensitivity to light and sound. Triptans are taken in the form of tablets, nasal sprays or through injection.
When treatment with triptans was introduced during the 1990s, they represented an entirely new way of treating migraine, and for many people the new treatments offered good pain-relieving effect. There are currently eight triptans that are approved for use against migraines, of which the three biggest sellers, sumatriptan (Imigran®, GSK), zolmitriptan (Zomig®, AstraZeneca) and rizatriptan (Maxalt®, Merck), make up the majority (around 80 percent) of the total triptan market3.
The patents behind these medications have expired, however, which has opened the door to a generic drugs market.
DHE
The second group, which is responsible for around 18 percent, comprises medications based on dihydroergotamine (DHE). DHE is a semi-synthetic product that has proven to be an effective alternative for those migraine patients who do not respond to or cannot take triptans, such as patients with cardiovascular diseases.
Proportion of sales value³
More distribution forms
Even though alternatives are now available in the form of nasal sprays and injections, traditional tablets still represent the most common form of distribution for medications for treating migraine. One challenge for medications in tablet form, however, is that their effect is limited or completely lost if the migraine attack causes vomiting before the substance reaches the intestines, where it is normally absorbed into the blood. Even if there is no vomiting, the effect of the medications can be impaired as a result of the fact that activity in the gastro-intestinal tract is reduced during a migraine attack, delaying absorption in the intestines. Alternative distribution forms have clear benefits in this respect. Their disadvantages include the fact that they are often more complicated for the individual patient to handle and use. Injection provides rapid, reliable effect, but many patients find injecting themselves to be unpleasant. Nasal sprays also provide relatively rapid effect, but some patients find them unpleasant and may experience vomiting when the dose runs from the sinuses into the throat1.
High anticipated growth
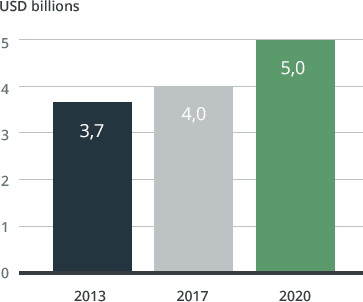
A high level of growth in the sale of medications for treating migrain is expected in the future. In 2017, global sales are expected to reach around USD 4 billion, before increasing to more than USD 5 billion annually after 20203. The driving forces behind this anticipated development include innovation in the delivery of medications, which means that established and generic medications can be administered more quickly and more reliably. Migraines are currently underdiagnosed and undertreated. Increased awareness of migraine among doctors and patients, combined with innovations in the delivery of clinically proven and marketed medications, will drive growth in this area as a large proportion of the medication patents have expired.
Anticipated growth in medications to treat migraine³
Recently completed deals
Now that the patents supporting leading medications have expired, investments in continued product development have increased significantly. A number of transactions have been conducted in recent years. These have been high value deals, indicating a positive view of the future earnings potential. In January 2014, NuPathe licensed out its technique for distributing sumatriptan via plasters to Teva. The cost of the licence amounted to USD 144 million4. In January 2013, MAP Pharmaceuticals acquired the Levadex® programme (DHE in inhalers) from Allergan for an estimated USD 958 million5.
1) www.migraine.com
2) The Global Market for Pain Management Drugs and Devices, 2013, BCCResearch
3) Global Migraine Drugs Market – 2015-2019, 2014, Technavio Research
4) http://www.bloomberg.com/news/articles/2014-01-21/teva-to-acquire-nupathe-for-144-million-outbidding-endo
5) www.allergan.com

Klaria receives funding for the KL-00119 project from the EU's Horizon 2020 program for research and development in accordance with grant no. 829615.
